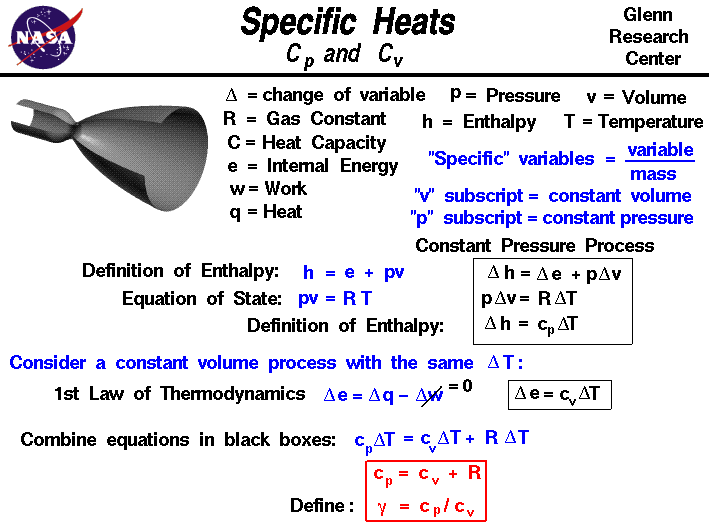
Heat Capacity Ratio Nitrogen . As the temperature of the gas returns to ambient the pressure rises. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. ratios of specific heat for gases with constant pressure and volume processes. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: The extent of the pressure. rearranging we have p=nrt/v. The values above apply to undissociated states. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats.
from exogeegww.blob.core.windows.net
ratios of specific heat for gases with constant pressure and volume processes. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: As the temperature of the gas returns to ambient the pressure rises. The extent of the pressure. rearranging we have p=nrt/v. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. The values above apply to undissociated states. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats.
Heating Capacity Of Heat Pump Formula at Lindsey Hazelton blog
Heat Capacity Ratio Nitrogen The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. ratios of specific heat for gases with constant pressure and volume processes. As the temperature of the gas returns to ambient the pressure rises. rearranging we have p=nrt/v. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The values above apply to undissociated states. The extent of the pressure.
From heat-transfer-thermodynamics.blogspot.com
Heat Transfer and Applied Thermodynamics Specific Heat Ratio Heat Capacity Ratio Nitrogen As the temperature of the gas returns to ambient the pressure rises. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. ratios of specific heat for gases with constant pressure and volume processes. rearranging we have p=nrt/v. The extent of the pressure. The values above apply. Heat Capacity Ratio Nitrogen.
From www.researchgate.net
(a) Pressure dependence of the heat capacity ratio γ = c p /c v for Heat Capacity Ratio Nitrogen 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. The values above apply to undissociated states. As the temperature of the gas returns to ambient the pressure rises. ratios of specific heat for gases with constant pressure and volume processes. the specific heat (= specific heat. Heat Capacity Ratio Nitrogen.
From www.numerade.com
SOLVED Compute the specific heat capacity at constant volume of Heat Capacity Ratio Nitrogen The extent of the pressure. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: rearranging we have p=nrt/v. The values above apply to undissociated states. ratios of specific heat for gases with constant pressure and volume processes. . Heat Capacity Ratio Nitrogen.
From www.slideserve.com
PPT Determining the Specific Heat Capacity of Air PowerPoint Heat Capacity Ratio Nitrogen As the temperature of the gas returns to ambient the pressure rises. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. rearranging we have p=nrt/v. The values above apply to undissociated states. The extent of the pressure. The specific heat capacity of a substance, usually denoted by. Heat Capacity Ratio Nitrogen.
From www.youtube.com
Equipartition Theorem Internal Energy (U) and Heat Capacity (Cv) L6 Heat Capacity Ratio Nitrogen The extent of the pressure. rearranging we have p=nrt/v. As the temperature of the gas returns to ambient the pressure rises. The values above apply to undissociated states. ratios of specific heat for gases with constant pressure and volume processes. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a. Heat Capacity Ratio Nitrogen.
From exogeegww.blob.core.windows.net
Heating Capacity Of Heat Pump Formula at Lindsey Hazelton blog Heat Capacity Ratio Nitrogen The extent of the pressure. rearranging we have p=nrt/v. As the temperature of the gas returns to ambient the pressure rises. The values above apply to undissociated states. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The specific heat capacity of a substance, usually denoted by. Heat Capacity Ratio Nitrogen.
From www.researchgate.net
Density of heat flux into the liquid nitrogen as a function of Heat Capacity Ratio Nitrogen The extent of the pressure. rearranging we have p=nrt/v. The values above apply to undissociated states. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: ratios of specific heat for gases with constant pressure and volume processes. . Heat Capacity Ratio Nitrogen.
From www.researchgate.net
Density of nitrogen with change in temperature and at different Heat Capacity Ratio Nitrogen The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: The extent of the pressure. ratios of specific heat for gases with constant pressure and volume processes. The values above apply to undissociated states. the specific heat (= specific. Heat Capacity Ratio Nitrogen.
From www.toppr.com
A gaseous mixture contains equal number of hydrogen and nitrogen Heat Capacity Ratio Nitrogen As the temperature of the gas returns to ambient the pressure rises. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The extent of the pressure. . Heat Capacity Ratio Nitrogen.
From www.doubtnut.com
Calculate the difference between two specific heats of 1 g of nitrogen Heat Capacity Ratio Nitrogen The values above apply to undissociated states. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. ratios of specific heat for gases with constant pressure and. Heat Capacity Ratio Nitrogen.
From www.chegg.com
Solved Experiment Ratio Heat Capacities Table.1 Data (The Heat Capacity Ratio Nitrogen The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: rearranging we have p=nrt/v. ratios of specific heat for gases with constant pressure and volume processes. the specific heat (= specific heat capacity) at constant pressure and constant. Heat Capacity Ratio Nitrogen.
From classes.oc.edu
THE LATENT HEAT OF VAPORIZATION OF NITROGEN Heat Capacity Ratio Nitrogen ratios of specific heat for gases with constant pressure and volume processes. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: rearranging we have p=nrt/v. The extent of the pressure. 47 rows in thermal physics and thermodynamics,. Heat Capacity Ratio Nitrogen.
From www.chegg.com
Solved Compute the average specific heat at constant Heat Capacity Ratio Nitrogen 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. The values above apply to undissociated states. ratios of specific heat for gases with constant pressure and volume processes. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample. Heat Capacity Ratio Nitrogen.
From haipernews.com
How To Calculate Heat Capacity Ratio Haiper Heat Capacity Ratio Nitrogen 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. ratios of specific heat for gases with constant pressure and volume processes. As the temperature of the. Heat Capacity Ratio Nitrogen.
From www.youtube.com
Ratio of Specific Heat Capacities YouTube Heat Capacity Ratio Nitrogen the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The values above apply to undissociated states. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: 47 rows. Heat Capacity Ratio Nitrogen.
From www.chegg.com
Solved Heat Capacity of Nitrogen Gas 20200308 4 of 6 » Heat Capacity Ratio Nitrogen 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. As the temperature of the gas returns to ambient the pressure rises. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of. Heat Capacity Ratio Nitrogen.
From www.researchgate.net
The temperature dependences of the idealgas specific heat ratio γ0 of Heat Capacity Ratio Nitrogen As the temperature of the gas returns to ambient the pressure rises. rearranging we have p=nrt/v. ratios of specific heat for gases with constant pressure and volume processes. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance, divided by the mass of the sample: . Heat Capacity Ratio Nitrogen.
From www.toppr.com
The Molar heat capacities of nitrogen at constant pressure and constant Heat Capacity Ratio Nitrogen As the temperature of the gas returns to ambient the pressure rises. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The extent of the pressure. The specific heat capacity of a substance, usually denoted by or , is the heat capacity of a sample of the substance,. Heat Capacity Ratio Nitrogen.